Titration Ratio . For example, you can use. Reviewed by bogna szyk and steven wooding. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution.
from www.chemistryscl.com
a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. Reviewed by bogna szyk and steven wooding. For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the.
Titrimetry, Titration Classifications, Standard solutions, Equivalence
Titration Ratio a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. For example, you can use. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Reviewed by bogna szyk and steven wooding. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Ratio titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a technique used to work out the concentration of an unknown solution based on its. Titration Ratio.
From chem.libretexts.org
Complexation Titration Chemistry LibreTexts Titration Ratio titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. For example, you can use. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Reviewed by bogna szyk and steven wooding. titration is a way of measuring. Titration Ratio.
From mavink.com
Titration Labeled Titration Ratio titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. a titration is a technique used to work out the concentration of an unknown. Titration Ratio.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Ratio titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Reviewed by bogna szyk and steven wooding. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a technique used to work out the concentration. Titration Ratio.
From www.reddit.com
Titration Ratios at the Equivalence Point r/Mcat Titration Ratio a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Reviewed by bogna szyk and steven wooding. titration. Titration Ratio.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Ratio a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. Reviewed by bogna szyk and steven wooding. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the above equation works only for. Titration Ratio.
From www.youtube.com
9.1 The Winkler method (SL) YouTube Titration Ratio a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the above equation works only for neutralizations in which there is a 1:1. Titration Ratio.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Ratio a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. Reviewed by bogna szyk and steven wooding. titration calculations for a neutralization. Titration Ratio.
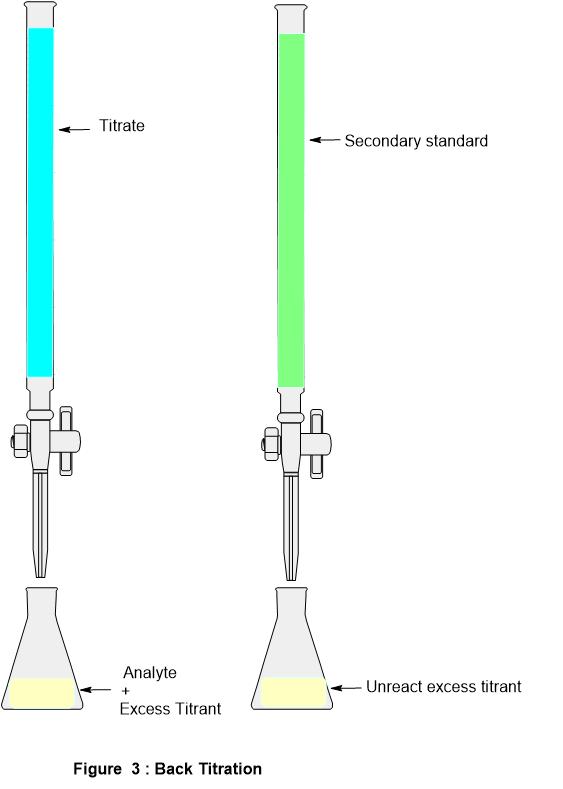
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Titration Ratio Reviewed by bogna szyk and steven wooding. For example, you can use. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. a titration is a technique used. Titration Ratio.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Ratio Reviewed by bogna szyk and steven wooding. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. a titration is a technique used to work out the concentration. Titration Ratio.
From hicensvanderkruijs.blogspot.com
The Graph Shows The Titration Curves Of A 1M Solution / Consider The Titration Ratio the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Reviewed by bogna szyk and steven wooding. For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration calculations for a neutralization reaction. Titration Ratio.
From mungfali.com
Acid Base Titration Calculation Titration Ratio a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. For example, you can use. Reviewed by bogna szyk and steven wooding. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration. Titration Ratio.
From labthink.netlify.app
What is titration and how does it work Titration Ratio titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the. Titration Ratio.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Ratio a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Reviewed by bogna szyk and steven wooding. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a technique used to work out the. Titration Ratio.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Titration Ratio Reviewed by bogna szyk and steven wooding. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. a titration is a laboratory technique. Titration Ratio.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Ratio Reviewed by bogna szyk and steven wooding. For example, you can use. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a technique used. Titration Ratio.
From www.chegg.com
Solved The titration curve below shows the results of the Titration Ratio a titration is a technique used to work out the concentration of an unknown solution based on its chemical reaction with a solution of known. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between. Titration Ratio.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Titration Ratio Reviewed by bogna szyk and steven wooding. titration calculations for a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. the above equation works only for neutralizations in which there is a. Titration Ratio.